The clinical trial was commissioned by Urathon Ltd and conducted by a team led by staff from the School of Biomedical Sciences, University of Leeds, who acted as independent researchers. It should be noted that all data was held by and analysed by the research team and that the team are totally independent from Urathon. The aim of this trial was to examine the accuracy and efficacy of the CT3 Yuwell continuous glucose monitor.
Dr Andrea Utley
School of Biomedical Sciences, University of Leeds (Research Lead)
Dr Andrea Utley is a Reader in Motor Control, Learning, and Development in the School of Biomedical Sciences at the University of Leeds. She is the Schools lead on impact and innovation and has led a number of clinical trials in health care related studies. In 2016 Dr Utley was awarded The Cutlers’ Surgical Prize by the Royal College of Surgeons for the design of the Yorkshire Micro Forcep. In addition, she was a finalist in the 2013 NHS innovation awards.
Dr Matthew Lancaster
School of Biomedical Sciences, University of Leeds (Co-investigator)
Dr Matthew Lancaster is an Associate Professor in Exercise Physiology in the School of Biomedical Sciences at the University of Leeds. His research is in cardiovascular health with a focus on how ageing alters the heart leading to problems such as arrhythmias and reduced exercise capacity. He has a depth of experience with physiological measures including the measurement of blood glucose as well as statistical analysis of multiple interacting factors and their impact on the cardiovascular system. He is a member of the Physiological Society and American Heart Association and the lead statistician on the project.
Dr Pippa Garner
School of Pharmacy & Medical Sciences: Faculty of Life Sciences, University of Bradford (Co-investigator)
Dr Garner specialise in the regenerative medicine and the techniques that are now being used to help the body to repair itself. She has a range of experience conducting research on medical devices and has worked with a range of research teams with the focus on medical and biological engineering.
Dr Hope Edwards
School of Biomedical Sciences, University of Leeds (Researcher)
Hope Edwards completed her MSci in Biological Sciences at The Royal Veterinary College, attaining First-Class Honours. Her previous research at the University of Bradford involved investigating Sarcopenia and the effectiveness of nutritional and exercise intervention as a therapeutic for disease.
The study gained full ethical permission from the Faculty of Biological Sciences ethics committee (Application 2044). In addition, the study was registered with the Open Science Framework as a clinical trial (https://osf.io/5ywr9).
Participants
A total of n=25 participants were recruited for the 10-day study including n= 13 males and n= 12 females. All participants were aged between 37 and 76 years old with a mean age of 57.8. The study population was made up of Type 2 diabetics n=25. 16% of the participants identified as minority ethnic. Body Mass Index (BMI) was also measured with participants ranging from 21.7 to 49.1 with a mean of 30.7.
Pilot Test
All procedures were tested in a pilot study to ensure that participants were clear on how to: attach and connect the device, administer the finger prick test, record their results. We also check that the workshops that demonstrated how to attach and use the device were clear and appropriate. The pilot tests revealed that all procedures were clear and appropriate.
Procedures
The participants were asked to monitor their glucose levels for 10 days using two devices. One device was attached to the skin and remained in position for the duration of the study (CT3 Yuwell Continuous Glucose Monitor). The second device was the Accu-chek glucose finger prick test. Participants were asked to take measurements on three occasions per day, before breakfast, before lunch, and before their evening meal. Participants were given a log book in which to record their glucose levels. At the start of the study participants were invited to the University of Leeds so that the use of the device could be demonstrated, consent taken and the participants given the opportunity to ask questions. It was made clear that they have the right to withdraw from the study at any point without giving a reason.
The sensor could be placed on the upper arm or stomach and participants were allowed to choose which site they would prefer. All participants placed the sensor on the upper arm. The placement of the sensor and the connection of the transmitted were demonstrated to all participants and the connection via Bluetooth from the transmitter to the phone app of the participant was checked by the researcher.
Participants were instructed to carry on with their normal daily activities and it was made clear that the device could remain in place when showering, swimming, or exercising. No changes to the participant’s normal daily routine was required.
CT-3 Yuwell (Continuous Glucose Monitoring Device)
The device uses mobile Bluetooth and a phone app to report glucose levels that are continuously gathered by the sensor and sent to the phone via the transmitter. The device provided unlimited data storage, readings every 3 minutes, and bluetooth technology that synchs directly to the participants phone or tablet.
Accu-chek testing (Finger prick blood testing equipment)
The accu-chek devices were checked for accuracy using a control solution kit (Roche, Germany). The kit contained two control solutions, Control 1 and Control 2. A test strip was inserted into the accu-chek device and either Control 1 or Control 2 were applied to the strip. The device was subsequently instructed which control solution was being tested and the screen displayed 'OK' if the validation was correct. A high level of accuracy was found for the accu-chek devices sampled.
A full statistical analysis of the data has been undertaken to examine the accuracy of the CT3 Yuwell CGM compared to the accu-chek finger prick test and to look at the trends of glucose levels over time. It should be noted that the analysis revealed no differences when comparing gender, BMI, and ethic background.
Results Summary
The results have shown good accuracy for the Yuwell device compared to a fingertip blood glucose test (accu-chek). We have obtained a MARD of 7.9% and other statistical measures show a very good agreement between the Yuwell CGM and blood glucose (see figure 1 and 3). In addition the reliability of the sensors accuracy is good across the typical range of glucose concentrations (see figure 2). The Yuwell CGM therefore trends well- spikes in blood glucose are also identified by Yuwell device. Participants gave favourable feedback on the Yuwell device and found it very motivating in terms of monitoring their glucose levels.
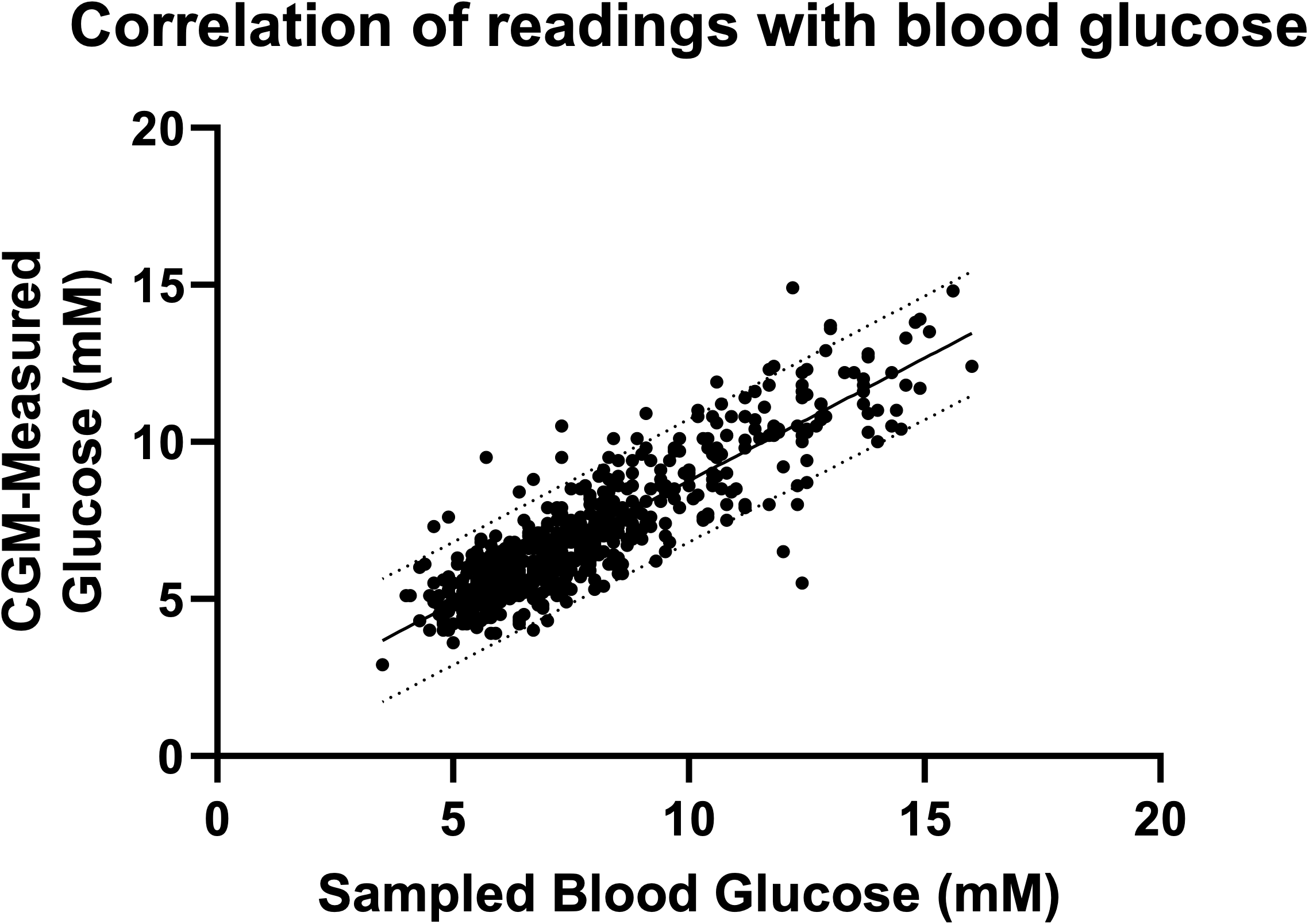
Figure 1. Linear correlation between pairs of CGM and blood measured glucose values. Solid line shows the line of best fit to the total data. Dashed line show 95% confidence intervals of agreement.
Pearson correlation gives a correlation coefficient of 0.88 (p<0.001) showing a strong, significant agreement between measures. Across the full data range there is very good agreement between the CGM and blood glucose (MARD 7.9%).
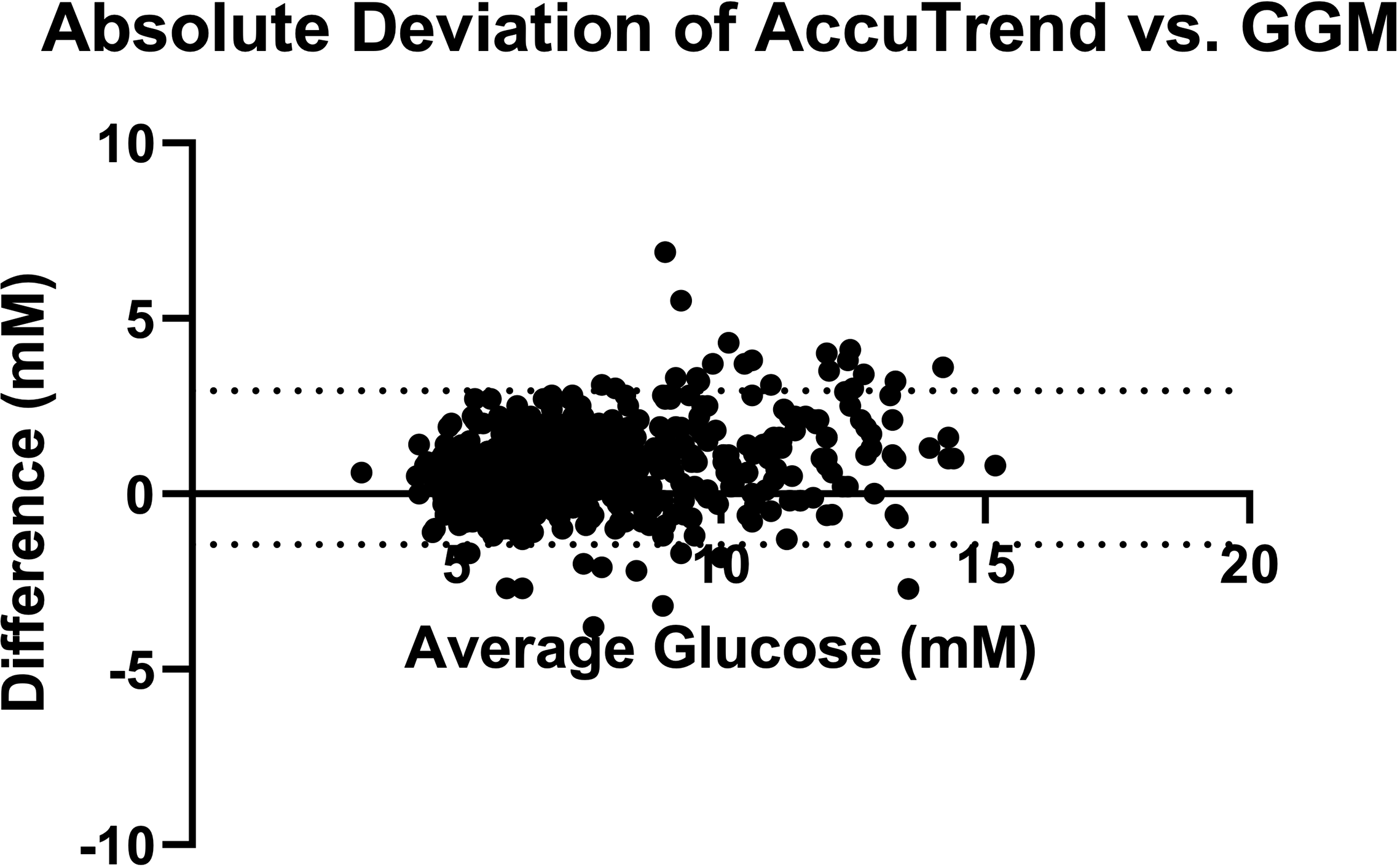
Figure 2. Bland-Altman analysis of agreement between the measurement approaches. Difference between CGM and blood analysis of glucose vs. glucose concentration.
This analysis identifies any concentration-dependent or systematic difference between the two measurement approaches. There is no significant concentration dependent-gradient in variation so reliability of the sensor is preserved across the typical range of glucose concentrations. The distribution does however show a bias between the two with an offset of 0.7 mM, although with a standard deviation of 1.1 mM and broad limits of agreement so this bias does not reach significance. Overall, this indicates good agreement and no significant difference between measures, therefore the device trends well.
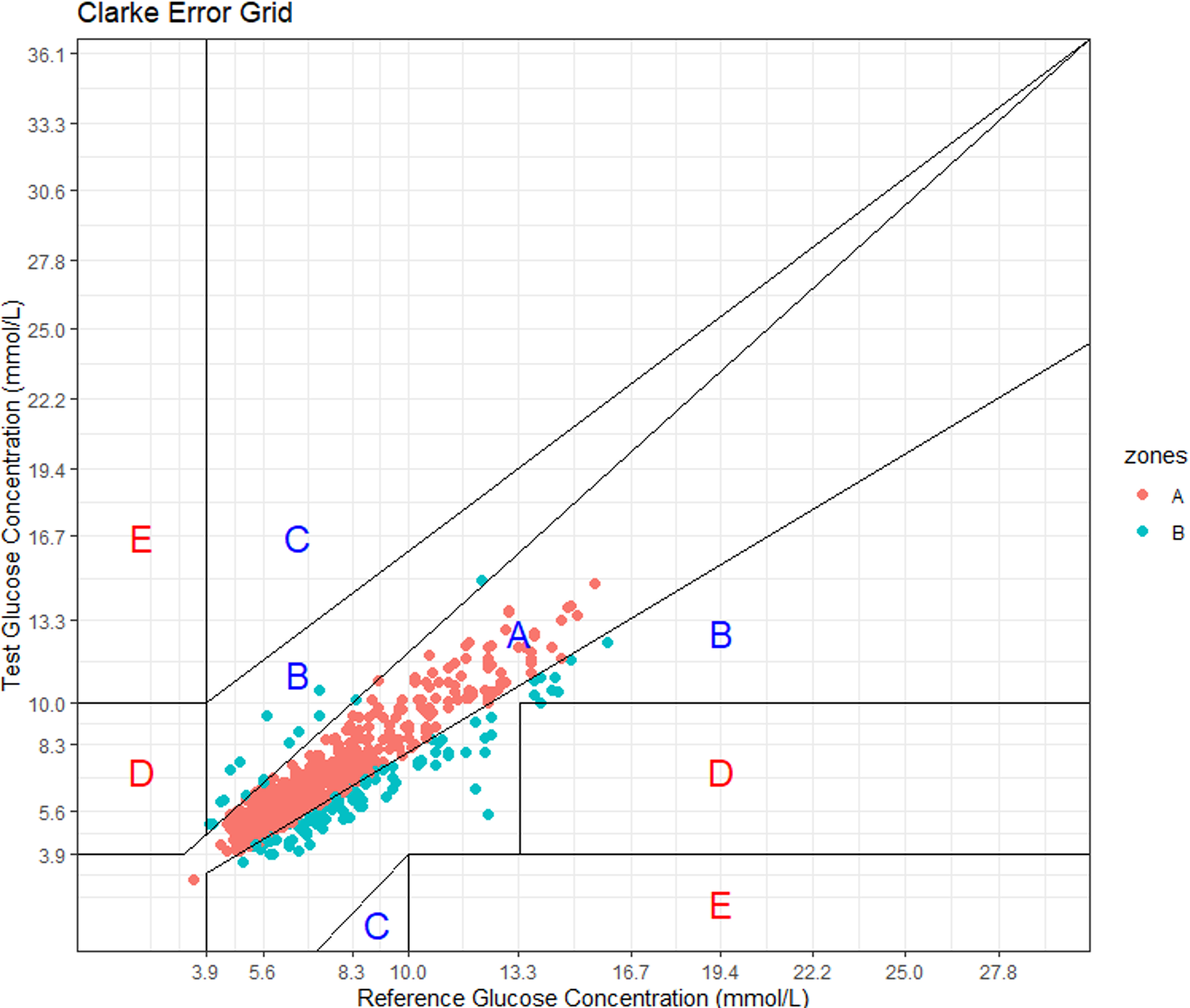
Figure 3. Plot of paired values on a colour-coded error grid. Region A indicates points within 20 %; B = outside 20 % but not significantly different; C and D are significant but limited in range misses; E are significant far misses.
Distribution of data between regions (percentages):
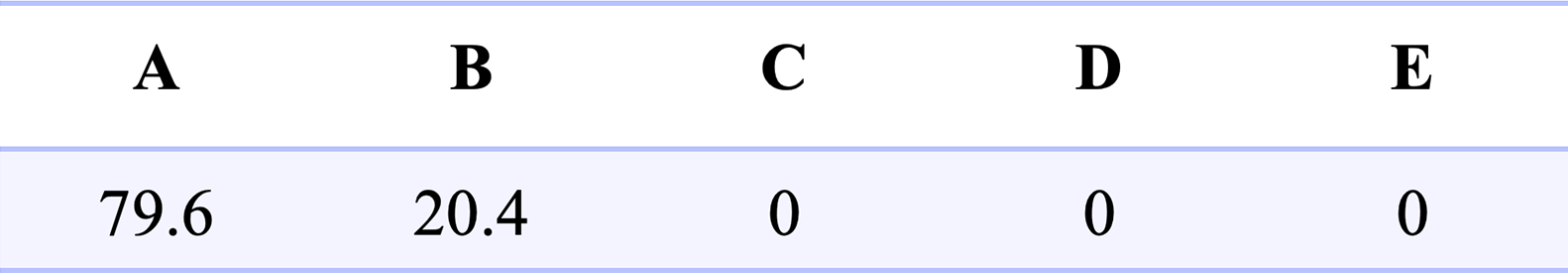
Figure 3 shows the Clarke Error Grid Analysis of Data Agreement. Region A indicates values within 20% of blood values. A and B together encapsulate no significant difference between measurement approaches and includes 100 % of data points. The distribution does show the apparent slight bias towards the low side with a number of measures falling into the lower B classification. However, overall there is a very good agreement across the range of distributions.
There are some very encouraging aspects of the Yuwell CGM device.
All of the above is especially encouraging as the device was tested in ‘free living conditions’.
Should you need further information about the clinical trial please email A.Utley@leeds.ac.uk
Data submitted for publication




By signing up, you agree to receive emails from Urathon Europe Ltd regarding Yuwell Anytime. We will treat your personal data with care and in accordance with our Privacy Policy, which you can review here. You can unsubscribe from our emails at any time by clicking the 'unsubscribe' link at the bottom of our emails.